Refueling emissions are generated from all liquid fuel storage and delivery systems when volatile liquids, like gasoline, are transferred from a storage tank to a receiving tank. Whether from tanker ship to terminal or underground storage to automobile -- vapor emissions escape into the atmosphere costing valuable product and damaging the environment.
The vapors initially present in the receiving tank are displaced from the headspace by the incoming liquid during the filling process. In the case of vehicle refueling, these "refueling emissions" can be directed back to the storage tank headspace or to an activated carbon canister located on the vehicle. If the refueling emissions are not directed back to the headspace of the storage tank, atmospheric air enters the storage tank to equalize the negative pressure caused by the outflow of liquid. The air dilutes the concentration of vapors in the storage tank headspace below the natural equilibrium saturation value. This results in the evaporation of additional liquid from the storage tank to reestablish equilibrium in the headspace.
The re-equilibration of the storage tank vapor space is a dynamic process that occurs over time and occurs during and after the liquid dispensing event. In gasoline retail outlets, the duration between bulk tanker deliveries provides ample time for re-equilibration of the storage tank headspace to occur. Re-equilibration results in the evaporation of hydrocarbons and corresponding pressure increases within the headspace of the storage tank. (Note: 1 gallon of liquid gasoline evaporates to ~520 gallons of vapor @ 40% hydrocarbon concentration). Increased pressure in the tank leads to vapor emissions through pressure/vacuum relief vents and through leaks in the vapor pathway. Without additional processing, evaporative emissions result in substantial product loss, environmental emissions, and health & safety hazards. These evaporative emissions are costly. Unfortunately, the new ORVR systems, which are effective in reducing refueling emissions, actually increase evaporative emissions from storage tanks.
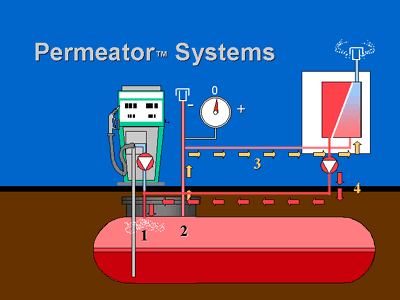
The gas separation membranes used in the PERMEATOR consist of an extremely thin, selectively permeable, polymeric film attached to a porous support structure. Membrane films are integrated into modules to provide maximum surface area per unit volume of pressure housing. Unlike conventional particle filters that separate materials based on physical size differences, ARID's vapor separation membranes separate compounds based on differences in the solubility and diffusivity of specific molecules. Hydrocarbon molecules pass through, or permeate the thin polymer film more rapidly than other molecules and are returned to the underground storage tank (Figure 1). Molecules such as oxygen and nitrogen (air) are much slower permeators, and are "rejected" by the membrane film and vented to the atmosphere. The difference in permeation rates between hydrocarbon and air molecules allows for the "selective" separation of gasoline vapors from air.
Figure 1
ARID's membrane-based system provides a simple, safe and economical solution that eliminates emissions of gasoline vapors at dispensing facilities. Removing excess air from storage tanks prevents over pressurization from occurring and virtually eliminates fugitive emissions. Storage tanks can be controlled to keep them at a slight negative pressure relative to atmospheric pressure to eliminate fugitive and vent emissions.